Introduction
The
spatial arrangement of atoms within a material plays an important role in
determining the properties of the material.
Solids
can be classified into three major types
1. Amorphous
An
amorphous solid does not have a well-defined structure; it is formless. There
is no recognizable long-range order in the positioning of atoms within the
material. The atomic arrangement in any given section of an amorphous material
will look different from the atomic arrangement in any other section of the material
(see Fig. 1(a)).
2. Polycrystalline
In
a polycrystalline solid there are many small regions each having an
well-organized structure but differing from its neighboring regions and
separated by grain boundaries (see Fig. 1(b)).
3. Crystalline
In
a crystalline solid atoms are arranged in an orderly array that defines a
periodic structure called the lattice. A unit cell when repeated by itself
produces a crystalline solid. A unit cell contains complete information
regarding the arrangement of atoms, and hence the unit cell can be used to
describe the crystal structure. Simply stated, a unit cell is a small portion
of any given crystal that could be used to reproduce the crystal. A primitive
cell is the smallest unit cell possible (see Fig. 1(c)).
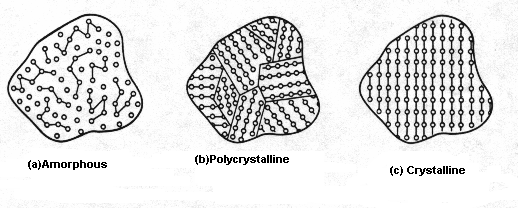
Fig.
1 General classification of solids based on the degree of atomic order: (a)
amorphous, (b) polycrystalline, and (c) crystalline.[7]